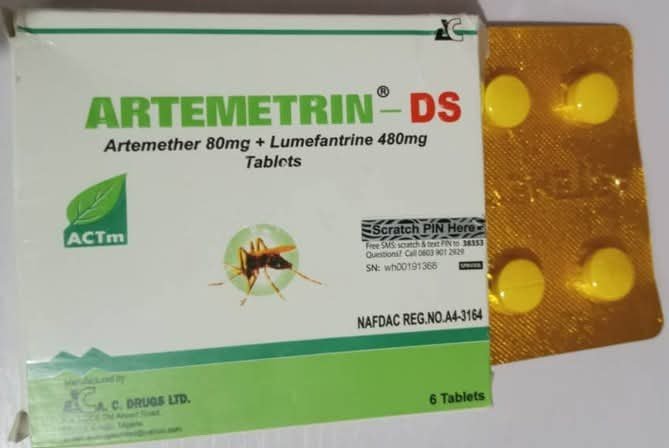
ABUJA, Nigeria – The National Agency for Food and Drug Administration and Control (NAFDAC) has raised alarm over the circulation of falsified malaria and antibiotic medicines in Nigeria, warning that the products pose a “grave threat” to public health.
In a statement on Wednesday, the agency identified two dangerous products—ARTEMETRIN DS (Artemether/Lumefantrine 80mg/480mg) and CIPROFIT 500 (Ciprofloxacin 500mg)—both falsely labelled as manufactured in Enugu.
Laboratory analysis revealed that ARTEMETRIN DS contained only 59.2% Artemether and 71.2% Lumefantrine, far below the internationally required 90–110% standard.
CIPROFIT 500 was found to have just 5.7% Ciprofloxacin, rendering it ineffective in treating bacterial infections.
NAFDAC stressed that the medicines, though purchased from a licensed vendor, were not listed in its official database. “The registration numbers on their packaging are fraudulent. These falsified medicines pose a serious threat as they cannot treat infections effectively,” the agency stated.
The agency has urged consumers, healthcare providers, and vendors to stop using or selling the drugs immediately and return any stock to the nearest NAFDAC office. It further advised: “If you or anyone you know experiences adverse reactions after using these products, seek urgent medical attention.”
Members of the public are encouraged to report suspicious drugs through NAFDAC’s toll-free line 0800-162-3322 or email sf.alert@nafdac.gov.ng.




