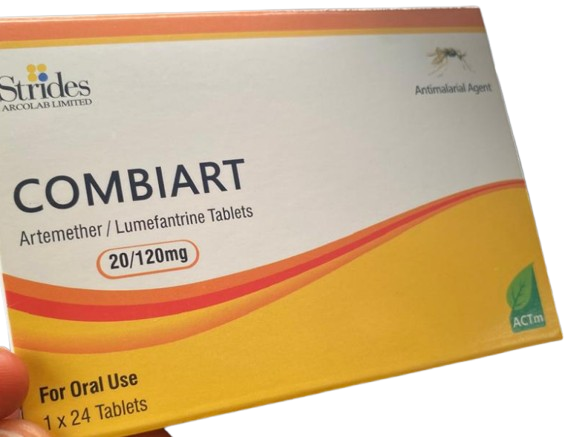
The National Agency for Food and Drug Administration and Control (NAFDAC) has released a public warning about the circulation of fake Artemether-Lumefantrine 20/120mg dispersible tablets, an essential drug for malaria treatment.
The affected batches include batch numbers 7225119, manufactured in June 2023 and February 2023, with expiration dates of May 2026 and June 2026. The genuine version of the medication is produced by Strides Arcolab Limited in Bangalore, India.
In a statement provided to Africa Health Report (AHR) on Thursday, NAFDAC instructed its zonal directors and state coordinators to enhance their monitoring efforts and eliminate the distribution of counterfeit medications.
“Importers, distributors, retailers, healthcare professionals, and caregivers are hereby advised to exercise caution and vigilance. All medical products must be obtained from authorized or licensed suppliers, and the products’ authenticity and physical condition should be carefully checked,” the agency stated.
NAFDAC emphasized the importance of strengthened vigilance within the supply chain to prevent the importation, distribution, and use of counterfeit medicines.
The public is encouraged to report suspected counterfeit drugs or adverse reactions through NAFDAC’s hotline at 0800-162-3322, via email at sf.alert@nafdac.gov.ng, or through the Med-Safety app, available on Android and iOS platforms.
“This alert will also be shared globally via the WHO Global Surveillance and Monitoring System (GSMS). “