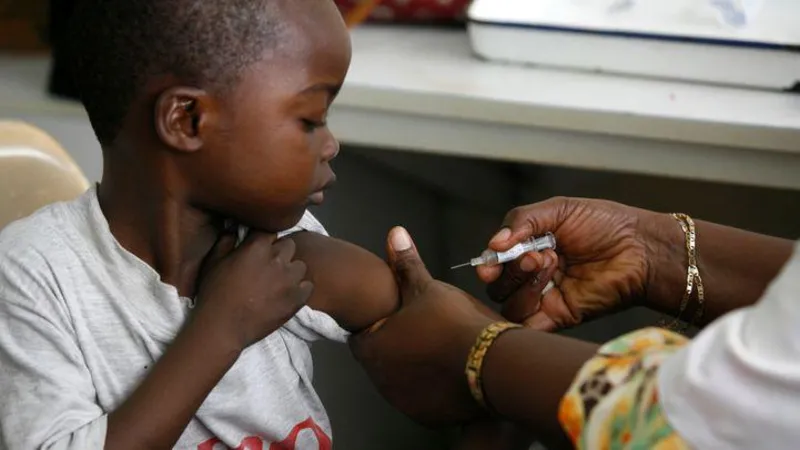
By Korede Abdullah in Lagos and Juliet Jacob in Abuja
More Nigerians have commended the significant breakthrough in the search for a suitable malaria treatment for newborns and babies resulting in manufacturing of the drug known as Coartem Baby or Riamet Baby, developed by Novartis in collaboration with the Medicines for Malaria Venture (MMV), a Swiss-based not-for-profit organisation.
MMV was initially backed by the British, Swiss and Dutch Governments, as well as the World Bank and the Rockefeller Foundation. The group announced on penultimate Monday that the first malaria treatment suitable for babies and very young children has been approved for use, adding its rollout is expected within the coming weeks.
The company’s chief executive, Vas Narasimhan, says this is an important moment.
“For more than three decades, we have stayed the course in the fight against malaria, working relentlessly to deliver scientific breakthroughs where they are needed most.
“Together with our partners, we are proud to have gone further to develop the first clinically proven malaria treatment for newborns and young babies, ensuring even the smallest and most vulnerable can finally receive the care they deserve.”
Although the company has yet to fix a definite roll out date for the drug, it said is likely to be rolled out in regions and countries with the highest rates of malaria within weeks.
“It’s expected to be rolled out in African countries within weeks”, Novartis reportedly stated.
Africa Health Report, AHR, reports that Novartis is planning to introduce it on a largely not-for-profit basis.
AHR correspondents sought reactions of a cross section of Nigerians following the development.
The comments by responders are presented below:
A Ray of Hope for Africa’s Youngest
A pediatrician in Lagos, Dr. Funke Adebayo, described it as “a life-saver.”
“For years, we have struggled with dosing older children’s medication for tiny babies. This is huge progress,” she told our reporter.
“Many parents don’t know how risky it is when we have no approved formulation for newborns. We’ve had to improvise and monitor very closely to avoid overdosing or underdosing — which can be fatal in severe malaria cases. Having a drug that is scientifically formulated for this vulnerable age group will give doctors and mothers so much relief and save countless lives,” the physician explained.
Mrs. Chioma Onuoha, a Lagos mother of a four-month-old, welcomed the news.
“We mothers are tired of losing babies to malaria. Anything that protects them from the mosquito bite and its danger is a blessing,” she told our correspondent.
A registered nurse, who works at a primary health centre in Yaba, Adeola Ogunyemi, called the new drug “a gap filler.”
“It will help us treat babies safely instead of guessing the dose of adult or older kids’ medication,” she said.
A father of two in Ikotun, Alimosho Local Government Area, Mr. Samuel Adediran, believes the new approval brings peace of mind.
“We’ve buried babies in my street because of malaria. If this works, many families will sleep better,” he said.
A Lagos-based pharmacist, Dr. Grace Eze, commended the innovation.
“The sweet flavor will make it less traumatic for mothers to give, and the correct dosing is a huge relief,” she said.
A community health worker in Igando, Mrs. Bola Azeez, urged quick deployment. “Now we need to make it affordable and available in all clinics and villages. No baby should die of malaria in this age,” she said.
Also responders in Abuja, Nigeria’s capital city described the news as a “A Lifesaver for Our Babies.”
“It is great news for Nigeria and for all health practitioners. Malaria remains a top killer of under-fives, and having a drug specifically designed for newborns will bridge a critical treatment gap,” said a medical doctor at the Kubwa General Hospital, Dr Nwachukwu.
At the Primary Healthcare Centre, Dutse-Buwari Area Council, a Nurse identified simply as Rose, said,
“I’m glad to hear this news. I just hope mothers will allow us give it to their babies. Our job is to create awareness and educate them about the new malaria drug.”
Mothers
A mother of a two-week-old in Kubwa, Halima,
“I can’t allow them to give my baby such drugs until I know more about it. I will do my research and my doctor has to recommend it before use.”
A mother working in Wuse, Blessing, commented:
“If the doctor says it’s safe, I will use it. Malaria is dangerous, especially for babies.”
Another mother residing in Bwari, Zainab, said:
“I lost a baby to malaria before. I will definitely use it if it is available in the hospital”.
Eight African nations also took part in the assessment and trials of the drug and they are expected to be among the first to access it.
These countries are; Nigeria, Burkina Faso, Côte d’Ivoire, Kenya, Malawi, Mozambique, Tanzania, and Uganda.
Swissmedic’s global health review and are expected to issue rapid local approvals under its Marketing Authorization for Global Health Products (MAGHP) procedure.
The death rate for malarial infections, particularly in sub-Saharan Africa is extremely high – over 76% of deaths occur in children under five years old.
Increase in death from malaria is further compounded in babies born with sickle cell disease, primarily due to a weak immune system.
Every year, about 30 million babies are born in areas with high malaria transmission.
In West Africa, the infection rates in infants under six months old can reach up to 18%. However, data on malaria in newborns is disturbing, because treatments specifically formulated for their age remain scarce as they are usually excluded from clinical trials, worsening their vulnerability.
Doctors have often had to improvise by splitting or crushing tablets meant for older children.
AHR reports that this is being condemned as a risky and imprecise practice that could lead to underdosing, overdose, or severe toxicity.