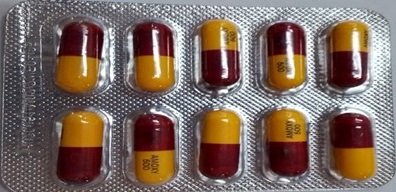
Nigeria’s drug regulator, the National Agency for Food and Drug Administration and Control (NAFDAC), has issued a public health warning after discovering falsified batches of Healmoxy Capsules 500mg, a commonly prescribed antibiotic, in neighboring countries.
The alarm was raised in NAFDAC Public Alert No. 17/2025, issued on Saturday, following reports that counterfeit consignments of the drug—purportedly manufactured by Indian pharmaceutical company Maxheal—were found in Cameroon and the Central African Republic (CAR).
According to the agency, the falsified Healmoxy capsules contain no active pharmaceutical ingredient (API), rendering them completely ineffective for treating bacterial infections.
“These capsules pose significant public health risks. Patients who take them believing they are being treated for bacterial infections could suffer severe consequences due to lack of treatment, potential toxicity, or antibiotic resistance,” NAFDAC warned.
Healmoxy 500mg is designed to contain Amoxicillin Trihydrate, a broad-spectrum penicillin-class antibiotic used to treat respiratory tract, urinary tract, gastrointestinal, and skin infections.
Though the Healmoxy brand is registered in Nigeria and reportedly imported by Nkoyo Chemist, located at 23 Agbonyi Street, Aguda, Surulere, Lagos, preliminary investigations by NAFDAC’s Post-Marketing Surveillance (PMS) directorate in Lagos found no evidence that the falsified products entered Nigeria through official channels.
“We are urging importers, pharmacists, hospitals, and the general public to remain vigilant,” a NAFDAC spokesperson told journalists. “Even if these batches were not officially imported, counterfeits can easily infiltrate our supply chains if caution is not maintained.”
NAFDAC advised that all medical products be sourced only from licensed suppliers, and that packaging and product information be carefully inspected for anomalies.
Healthcare professionals and the public are encouraged to report any suspicious medicines to NAFDAC through multiple channels:
Phone: 0800-162-3322, Email: sf.alert@nafdac.gov.ng, Med-Safety App: Available on Android and iOS
“The objective extends beyond safeguarding Nigerian consumers,” NAFDAC stated. “It is also about reinforcing the broader regional drug surveillance system. These counterfeit antibiotics endanger not only individual lives but also undermine efforts to combat antimicrobial resistance across West and Central Africa.”
NAFDAC’s warning aligns with growing concerns from the World Health Organization (WHO) over the rising spread of falsified medicines in African markets. The agency pledged to step up surveillance and collaborate with neighboring countries to curb the threat.