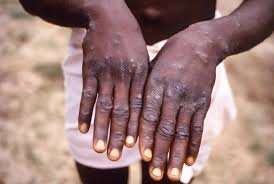
The World Health Organization (WHO) has made a significant breakthrough in the fight against mpox, approving the first mpox diagnostic test for emergency use. This milestone decision will greatly improve global access to mpox testing, particularly in countries struggling with outbreaks.
The approved test, Alinity m MPXV assay, manufactured by Abbott Molecular Inc., is a real-time PCR test that detects monkeypox virus DNA from human skin lesion swabs. This will enable laboratory and health workers to confirm suspected mpox cases efficiently and effectively.
The Need for Quick Testing
In Africa, over 30,000 suspected mpox cases have been reported this year, with the Democratic Republic of the Congo, Burundi, and Nigeria having the highest numbers.
However, limited testing capacity and delays in confirming cases have contributed to the virus’s continued spread. In the Democratic Republic of the Congo, only 37% of suspected cases have been tested this year.
How the Test Works
The Alinity m MPXV assay uses nucleic acid amplification testing (NAAT) to confirm the presence of the monkeypox virus. Lesion material is the recommended specimen type for diagnostic confirmation. Trained clinical laboratory personnel can use this test to detect DNA from pustular or vesicular rash samples.
WHO’s Response
Dr. Yukiko Nakatani, WHO Assistant Director-General for Access to Medicines and Health Products, hailed the approval as a significant milestone in expanding testing availability in affected countries. “Increasing access to quality-assured medical products is central to our efforts in assisting countries to contain the spread of the virus and protect their people, especially in underserved regions.”
Next Steps
The WHO has received three additional submissions for EUL evaluation and is engaging with other manufacturers to ensure a wider range of quality-assured diagnostic options. This will enable countries to procure critically needed tests through UN agencies and other procurement partners.